Set your Sights on Enhancing Value

A closer look at a breakthrough way of treating macular edema associated with uveitis1
Discover the first therapy to unlock the potential of the suprachoroidal space (SCS)1
A closer look:
The incidence and prevalence of ME associated with uveitis varies in the published literature and is believed to be underreported*
Epidemiology of Uveitis and ME Associated With Uveitis
0.017% to 0.052%
0.038% to 0.714%
91% of adult uveitis cases
94% 18-64 years age group
86% ≥65 years age group
2% to 32% (review of US and ex-US studies published from 1987 through 2016)4
9% (US administrative claims analysis of patients identified from October 2015 to March 2020)5†
US prevalence of ME associated with uveitis6*
30,879 to 77,198
Estimated US prevalence, based on expert opinion‡
ME, macular edema.
*Based on expert opinion.
†Based on commercially insured population.
‡Because there is no treatment that requires documentation of ME associated with uveitis, and there is not yet an ICD-10-CM diagnosis code specific to ME associated with uveitis, this condition is believed to be underreported.5 Therefore, reliance on expert opinion was used to estimate the US prevalence of ME associated with uveitis.
A closer look:
Uveitis with ME is associated with greater risk for vision loss compared with uveitis without ME5
Degree of Vision Loss Among Patients With Uveitis
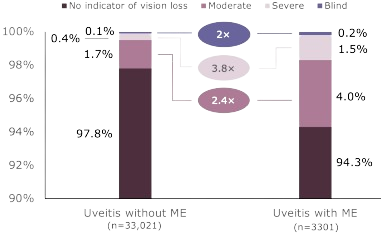
Patients with uveitis and ME experienced vision loss more frequently than did those with uveitis without ME.
A closer look:
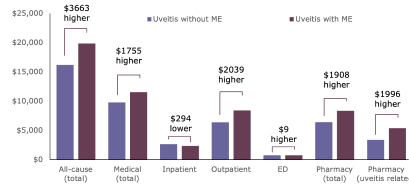
Patients with uveitis and ME incur substantially higher healthcare costs5
Total Costs: Uveitis Patients With and Without ME
Study design and objectives: Claims analysis (third-party claims database, MarketScan) to assess healthcare costs associated with noninfectious uveitis (NIU) with and without ME.
Population: 36,322 patients with an NIU diagnosis from October 2015 to March 2020.
Patients with ME incurred 23% higher costs compared with those without ME, driven by increased outpatient and pharmacy costs
ED, emergency department; PPPY, per patient per year; USD, United States dollars.
Macular edema is the leading cause of visual loss in uveitis.7
A closer look:
XIPERE® is the only corticosteroid indicated to treat UME by injecting into the suprachoroidal space1,8
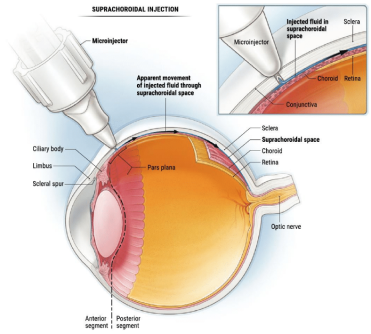
Targeted
Circumferential and posterior spreading of the drug following injection.9
Contained
By injecting into the SCS®, drug therapy can be concentrated in appropriate target tissues, minimizing exposure to the anterior segment.8
Accessible
XIPERE® treats uveitic macular edema by targeting the posterior segment.1,8
SCS, suprachoroidal space.
A closer look:
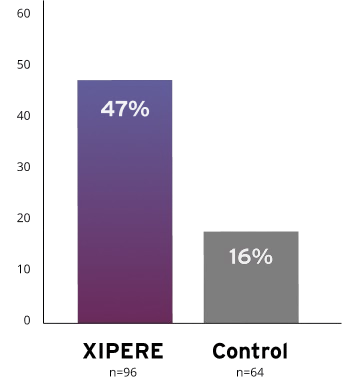
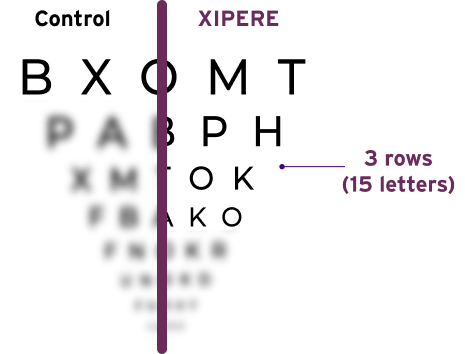
XIPERE® delivered a significant BCVA improvement of 15 ETDRS letters from baseline at Week 2410
47% of patients treated with XIPERE® achieved this compared with 16% of patients in the control group10
Proportion of patients who gained ≥15 ETDRS letters from baseline at Week 2410
A closer look:
Ocular Adverse Reactions Reported in ≥2% of Patients and Nonocular Adverse Reactions Reported by ≥5% of Patients1
| Adverse reaction, n (%) | XIPERE® (n=96) | Control (n=64) | |
|---|---|---|---|
| Ocular | |||
| Increased intraocular pressure, non-acute*† | 13 (14%) | 9 (14%) | |
| Eye pain, non-acute† | 11 (12%) | 0 | |
| Cataract‡ | 7 (7%) | 4 (6%) | |
| Increased intraocular pressure, acute*§ | 6 (6%) | 0 | |
| Vitreous detachment | 5 (5%) | 1 (2%) | |
| Injection site pain | 4 (4%) | 2 (3%) | |
| Conjunctival hemorrhage | 4 (4%) | 2 (3%) | |
| Visual acuity reduced | 4 (4%) | 1 (2%) | |
| Dry eye | 3 (3%) | 1 (2%) | |
| Eye pain, acute§ | 3 (3%) | 0 | |
| Photophobia | 3 (3%) | 0 | |
| Vitreous floaters | 3 (3%) | 0 | |
| Uveitis | 2 (2%) | 7 (11%) | |
| Conjunctival hyperemia | 2 (2%) | 2 (3%) | |
| Punctate keratitis | 2 (2%) | 1 (2%) | |
| Conjunctival edema | 2 (2%) | 0 | |
| Meibomianitis | 2 (2%) | 0 | |
| Anterior capsule contraction | 2 (2%) | 0 | |
| Chalazion | 2 (2%) | 0 | |
| Eye irritation | 2 (2%) | 0 | |
| Eye pruritus | 2 (2%) | 0 | |
| Eyelid ptosis | 2 (2%) | 0 | |
| Photopsia | 2 (2%) | 0 | |
| Vision blurred | 2 (2%) | 0 | |
| Nonocular | |||
| Headache | 5 (5%) | 2 (3%) | |
*Includes intraocular pressure increased and ocular hypertension.
†Defined as not occurring on the day of the injection procedure, or occurring on the day of the injection procedure and not resolving the same day.
‡Includes cataract, cataract cortical, and cataract subcapsular.
§Defined as occurring on the day of the injection procedure and resolving the same day.
A closer look:
Adding XIPERE® results in minimal budget impact6
The cost of XIPERE® for the treatment of ME associated with NIU is expected to be largely offset by lower healthcare resource utilization and costs
Estimated budget impact for XIPERE® from Year 1 to 5
$0.4K to $3.0K
per million members for commercial plans
$2.3K–$38.3K
per million members for Medicare plans
Estimated PMPM for XIPERE® remains less than a penny from Year 1 to 5
$0.0000–$0.0002
range for commercial plans
$0.0002–$0.0032
range for Medicare plans
PMPM, per member per month.
Based on economic modeling:
XIPERE® was cost-effective versus best supportive care (BSC) at willingness-to-pay (WTP) thresholds of $50,000 and $100,000 per QALY gained
– Sensitivity analysis showed very little uncertainty, with ICERs remaining below the $50,000 WTP threshold across all model parameters
Value-based pricing analysis suggests that the WAC for XIPERE® is ~27% below the price to exceed the WTP threshold of $50,000 per QALY gained
– The value-based price for XIPERE®—which yields an ICER of $50K per QALY—is $2274 per injection, compared to KAC of $1650 per injection
Results are derived from a patient-level Markov model over a 10-year time horizon comparing XIPERE® with BSC
| XIPERE® | BSC | |
|---|---|---|
| QALYs | 6.65 | 6.19 |
| Costs | $180,840 | $167,747 |
| Incremental QALYs of XIPERE® | 0.46 | |
| Incremental cost of XIPERE® | $13,093 | |
| ICER (cost per QALY gained) | $28,479 | |
| WTP Threshold | Value-based Price for XIPERE® | Current XIPERE® Price Is Lower by |
|---|---|---|
| @$50,00 per QALY gained | $2274 | ~27% |
| @$100,00 per QALY gained | $3725 | ~56% |
ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life year.
XIPERE® (triamcinolone acetonide injectable suspension) for suprachoroidal use is a corticosteroid indicated for the treatment of macular edema associated with uveitis.
Patients should be monitored following injection for elevated intraocular pressure. See Dosage and Administration instructions in full Prescribing Information.
To report SUSPECTED ADVERSE REACTIONS, contact Bausch + Lomb at 1-800-321-4576 or FDA at 1-800-FDA-1088 or visist www.fda.gov/medwatch
Click here for full Prescribing Information.
References:
1. XIPERE®. Prescribing information. Bausch & Lomb Inc. 2. Massa H, Pipis SY, Adewoyin T, Vergados A, Patra S, Panos GD. Macular edema associated with non-infectious uveitis: pathophysiology, etiology, prevalence, impact and management challenges. Clin Ophthalmol. 2019;13:1761-1777. 3. Thorne JE, Suhler E, Skup M, et al. Prevalence of noninfectious uveitis in the United States: a claims-based analysis. JAMA Ophthalmol. 2016;134(11):1237-1245. 4. Accorinti M, Okada AA, Smith JR, Gilardi M. Epidemiology of macular edema in uveitis. Ocul Immunol Inflamm. 2019;27(2):169-180. 5. Hariprasad SM, Joseph G, Gagnon-Sanschagrin P, et al. Healthcare costs among patients with macular oedema associated with non-infectious uveitis: a US commercial payer's perspective. BMJ Open Ophthalmol. 2021;6(1):e000896. Published online November 10, 2021. doi:10.1136/bmjophth-2021-000896 6. Data on file. 7. Levin MH, Pistilli M, Daniel E, et al. Incidence of visual improvement in uveitis cases with visual impairment caused by macular edema. Ophthalmology. 2014;121(2):588-595. 8. Chiang B, Jung JH, Prausnitz MR. The suprachoroidal space as a route of administration to the posterior segment of the eye. Adv Drug Deliv Rev. 2018;126:58-66. 9. Rai UDJP, Young SA, Thrimawithana TR, et al. The suprachoroidal pathway: a new drug delivery route to the back of the eye. Drug Discov Today. 2015;20(4):491-495. 10. Yeh S, Khurana RN, Shah M, et al. Efficacy and safety of suprachoroidal CLS-TA for macular edema secondary to noninfectious uveitis: phase 3 randomized trial. Ophthalmology. 2020;127(7):948-955.
XIPERE® (triamcinolone acetonide injectable suspension) for suprachoroidal use is a corticosteroid indicated for the treatment of macular edema associated with uveitis.
Patients should be monitored following injection for elevated intraocular pressure. See Dosage and Administration instructions in full Prescribing Information.
To report SUSPECTED ADVERSE REACTIONS, contact Bausch + Lomb at 1-800-321-4576 or FDA at 1-800-FDA-1088 or visist www.fda.gov/medwatch
Click here for full Prescribing Information.